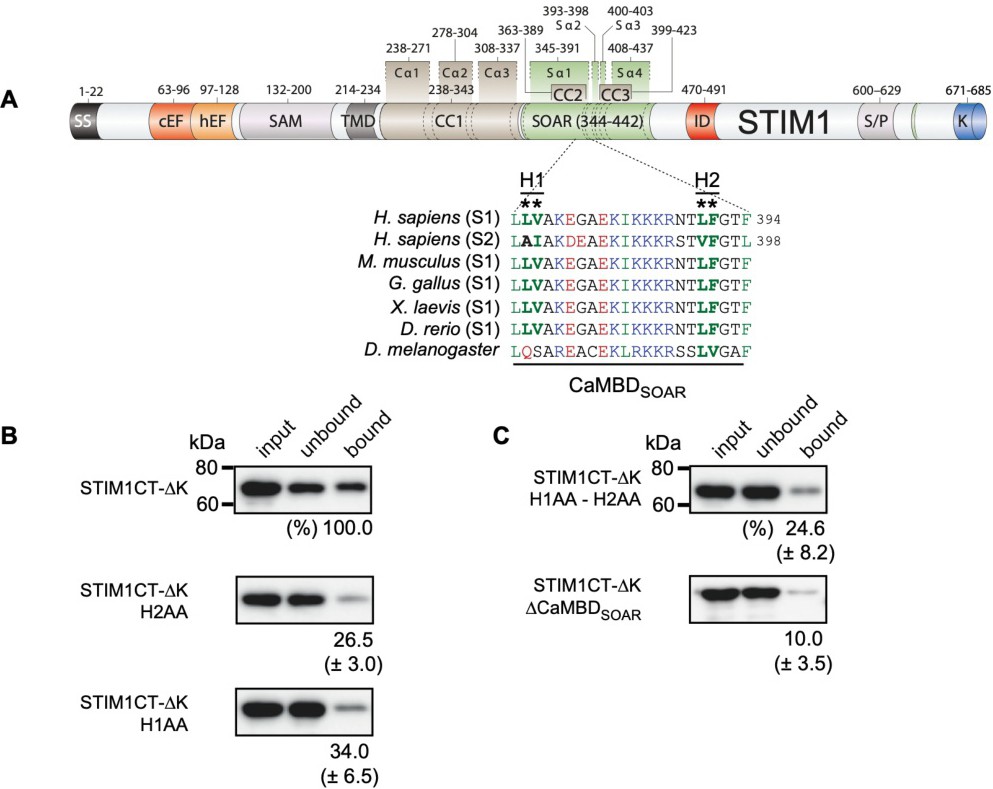
Fig. 1. Hydrophobic residues crucial for binding of Ca2+/Calmodulin to STIM1-Orai1 activating region. (A), Domain architecture of human STIM1 with sequence alignment of predicted CaM-binding domains (CaMBDs) of human STIM1 and STIM2 and STIM1 of different vertebrates and drosophila are shown. Conserved hydrophobic residues (H1 and H2) in STIM1-Orai1 activating region (SOAR) are indicated in bold green within CaMBDSOAR (residues 372-394) and are marked with asterisks. (B), Binding of GFP-tagged STIM1 C-terminus lacking the K-rich domain (STIM1CT-∆K) and STIM1CT-∆K with mutations of hydrophobic residues (L390A-F391A = H2AA and L374A-V375A = H1AA) in CaMBDSOAR to CaM beads. C, Binding of STIM1CT-∆K with combined H2AA and H1AA mutations and of STIM1CT-ΔK with deletion of CaMBDSOAR to CaM beads. The bound fraction of STIM1CT-∆K was set to 100 in all three experiments and the mean ▒ S.E.M. for the bound (eluted) fractions of all mutants is indicated.